This content is sponsored by Passy Muir
Prevalence
Annually, more than 100,000 patients in the United States experience medical events that require a tracheostomy, with 24 percent of those necessitating mechanical ventilation (Yu, 2010). It is estimated that by the year 2020, there will be over 600,000 patients requiring prolonged mechanical ventilation (Zilberberg, 2008). Most vented patients are on prolonged bedrest during hospitalization. In intensive care units (ICU) across the country, efforts are being made to implement early mobility programs as there is significant evidence indicating that many patients in intensive care units on prolonged mechanical ventilation experience a marked decline in functional status (Spicher, 1987). After one week of bedrest, muscle strength may decrease by as much as 20 percent with an additional loss of 20 percent each subsequent week of bedrest (Perme, 2009; Sciaky, 1994). Why is this significant to speech-language pathology?
Impact on Muscles of Respiration and Swallowing
The loss of strength extends to the muscles of respiration and the oropharyngeal musculature, which affects communication and swallowing (Griffiths & Jones, 1999), and muscle weakness is an independent predictor of pharyngeal dysfunction and symptomatic aspiration (Hermans & Van den Berghe, 2015). Patients with muscle weakness often have greatly reduced cough strength and poor control over their swallowing and upper airway (Griffiths & Jones, 1999). The implementation of early mobility and rehabilitation in the fields of physical and occupational therapy has shown positive outcomes in ICU patients (Adler, 2012). The same principles used to promote strength for mobility can be applied to strengthening the oropharyngeal and respiratory musculature to improve swallowing (Burkhead et al., 2007).
However, many times the medical team prefers to wait until the patient is weaned from the ventilator before consulting the speech-language pathologist on the basis that the patient is “too sick” to begin intervention for swallowing. Considering that research has shown how disuse leads to muscular atrophy and weakness in relatively short periods of time, waiting to intervene may contribute to significant dysfunction of the swallow mechanisms. Another factor with this patient population includes the alteration of the anatomy and physiology of the swallowing mechanisms that occur following a tracheostomy. The placement of a tracheostomy and prolonged mechanical ventilation with an inflated cuff causes a disconnect between the upper and lower airways. The lack of airflow through the upper airway has been shown to have negative changes causing reduced subglottic pressure (Eibling & Gross, 1996), decreased sensation to the pharynx and glottis (Eibling & Gross, 1996), reduced laryngopharyngeal reflex (Sasakai, Suzuki, Horiuchi, Masatoshi & Kirchner, 1997), decreased ability to manage secretions requiring more frequent suctioning (Siebens et al., 1993), decreased sense of taste and smell (Lichtman et al., 1995), inability to vocalize, increased aspiration risk, and muscle disuse and atrophy (Hermans & Van den Berghe, 2015; Griffiths & Jones, 1999). The weight of the tracheostomy tubing and an inflated cuff may decrease the range of motion of the hyolaryngeal mechanism by causing a tethering effect (Ding & Logemann, 2005; Bonanno, 1971). Furthermore, patients on mechanical ventilation may experience discoordination of breathing, which can negatively impact swallowing (Pringent et al., 2011).
Role of the Speech-Language Pathologist
The speech-language pathologist (SLP) is in a unique position to provide early intervention and rehabilitation to patients who are tracheostomized and on mechanical ventilation via early assessment for the use of a bias-closed, no-leak speaking valve (Passy Muir® Valve) for in-line use with the ventilator. Early intervention by the SLP should begin by identifying patients on mechanical ventilation who are candidates for trials using the Passy Muir Valve (PMV). The benefits of early assessment and implementation with the PMV are many. First and foremost, use of the PMV helps to restore the physiology of the upper airway to its more “normal” state by returning airflow through the upper airway during exhalation. This restoration of airflow to the upper airway allows evaluation of airway patency, vocal cord function, secretion management, swallowing, and communication skills. In many cases, voicing is restored, allowing the patient to communicate basic needs and participate in their daily care.
Early Intervention
With the negative impact on swallow function in this patient population, it is estimated that 50-87% of patients with tracheostomy aspirate, including silent aspiration (Elpern et al., 1987, 1994, 2000). Research suggests that tracheostomy speaking valves may positively impact swallowing function and rehabilitation (Blumenfield, 2011). Research also has demonstrated that early implementation of a swallowing rehabilitation program is feasible for patients on mechanical ventilation (Rodrigues et al., 2015). Physiologically, the bias-closed, no-leak valve design of the PMV allows for restoration of subglottic air pressure, which has been shown to decrease or prevent aspiration in some patients (Dettelbach et al., 1995). With the PMV in-line on mechanical ventilation, the SLP evaluates the integrity of the aerodigestive tract and more thoroughly assesses potential risk factors related to the function of the vocal cords, cough strength, and secretion management abilities. Furthermore, the redirection of airflow provides sensory stimulation to the oropharynx and can improve management of secretions, as well as improve taste and smell (Lichtman et al., 1995).
For those patients with dysphagia who are not candidates to begin an oral diet, PMV use can be instrumental in the swallow rehabilitation process. Almost all swallowing rehabilitation techniques require the restoration of subglottic pressure to achieve effective results. Almost all pharyngeal exercises, swallow maneuvers (such as the Mendelsohn and Supraglottic Swallow), and respiratory muscle strength training require a closed system and subglottic airway pressure. The rehabilitation process may begin with tasks as simple as getting the patient to relearn breathing through the upper airway again and working on managing secretions during PMV trials.
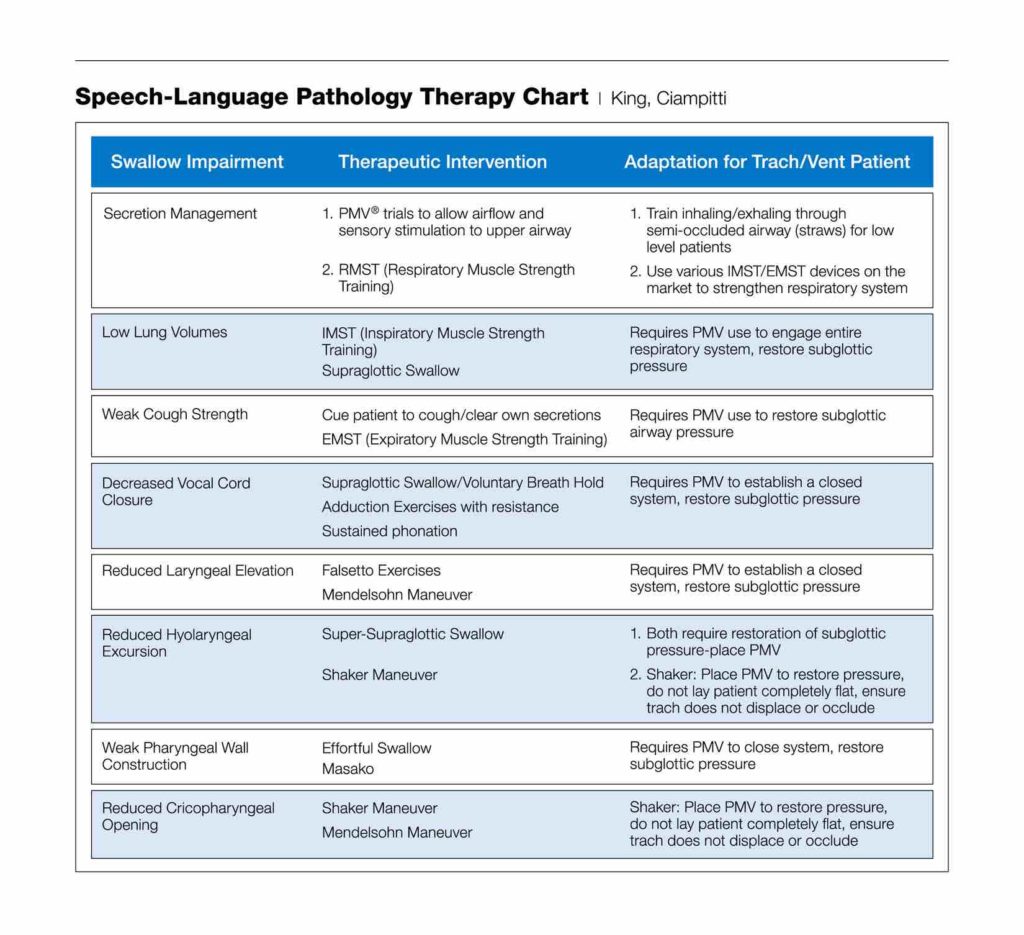
Many patients on prolonged mechanical ventilation also have decreased volumes and poor expiratory strength, which affect swallowing and may place a patient at higher aspiration risk (Gross et al., 2003). PMV use allows patients to participate in respiratory muscle strength training, which has been shown to improve both voicing and swallow function (Sapienza & Trocher, 2012). Although many patients successfully tolerate PMV trials on initial attempts, not all patients with tracheostomy will be immediately successful. Furthermore, not all swallowing function is improved by tracheostomy tube occlusion; therefore, other variables will need to be considered and evaluated (Donzelli et al., 2006). It is important to work with a multidisciplinary team to troubleshoot potential issues that may affect the ability to use the PMV, such as trach tube size, upper airway pathology, and underlying medical issues or co-morbidities, so that patients receive the best standard of care and are set up for success with the PMV.
Summary
Practices in the timing of speech-language pathology intervention for patients on mechanical ventilation vary across medical settings. Because early intervention addressing communication and swallow function has been shown to have a significant impact on patient outcomes, efforts should be made by speech-language pathologists to evaluate current protocols in ICUs and other medical settings to promote early intervention for patients with transient or chronic vent dependency.
Disclosures
Financial disclosure: The author is a prn consultant for Passy-Muir, Inc.
Non-financial disclosure: No relevant non-financial disclosures.
References
Adler, J. and Malone, D. (2012). Early Mobilization in the Intensive Care Unit: A Systematic Review. Cardiopulmonary Physical Therapy Journal, 23(1).
Blumenfield, L., Salgado M., Wade K., Dhupa A., Ling E., and Belafsky P. (2011). The effects of Tracheostomy Speaking Valve use on Disordered Swallowing. DRS Poster presentation.
Bonanno P.C. (1971). Swallow dysfunction after tracheostomy. Annals of Surgery, 174(1): 29-33.
Burkhead, L., Sapienza, C., and Rosenbek, J. (2007) Strength-training exercise in dysphagia rehabilitation; principles, procedures and directions for future research. Dysphagia, 22(3): 251-65.
Chen, Y., Jacobs, W.J., Quan, S.F., Figueredo, A.J., and Davis, A.H. (2011). Psychophysiological Determinants of Repeated Ventilator Weaning Failure: An Explanatory Model. American Journal of Critical Care, 20(4), 292-302. doi:10.4037/ajcc2011886
Dettelbach M.A., Gross R.D., Mahlmann J., and Eibling D.E. (1995). Effect of the Passy-Muir Valve on aspiration in patients with tracheostomy. Head Neck, 17(4): 297-302.
Ding R. and Logemann J.A. (2005). Swallow physiology in patients with trach cuff inflated or deflated: a retrospective study. Head & Neck, 27: 809-813.
Donzelli J.L. Brady S., Wesling M., and Theisen M. (2006). Secretions, occlusion status, and swallowing in patients with a tracheotomy tube; a descriptive study. Ear Nose Throat Journal, 85(12): 831-4.
Eibling D.E. and Gross R.D. (1996). Subglottic air pressure: A key component of swallowing efficiency. Annals of Otology, Rhinology & Laryngology, 105(4):253.
Elpern, E.H., Borkgren, M., Bacon, M., Gerstung, C. and Skrzynski, M. (2000). Effect of the Passy-Muir Valve on pulmonary aspiration in adults with tracheostomies. Chest, 116(4-S2):365S.
Elpern, E., Jacobs, E.R. and Bone, R.C. (1987). Incidence of aspiration in tracheally intubated adults. Heart & Lung, 105(2):527.
Elpern, E.H., Scott, M.G., Petro, L., and Ries, M.H. (1994). Pulmonary Aspiration in Mechanically Ventilated Patients With Tracheostomies. Chest, 105.2 (1994): 563-66.
Griffiths, R.D. and Jones, C. (1999). Recovery from intensive care. British Medical Journal, 319-427.
Gross, R.D., Atwood, C.W., Jr., Grayhack, J.P., and Shaiman, S. (2003). Lung volume effects on pharyngeal swallowing physiology. Journal of Applied Physiology, 95(6), 2211-2217.
Hermans, G and Van den Berghe, G. (2015). Clinical review: intensive care unit acquired weakness. Critical Care,19:274. [PubMed: 26242743]
Khalalia R., Zbidat W., Anwar K., Bayya A., Linton D.M., and Sviri S. (2011). Communication difficulties and psychoemotional distress in patients receiving mechanical ventilation. American Journal of Critical Care, 20(6): 470-9. doi:10.4037/ajcc2011989
Lichtman, S.W., Birnbaum I.L., Sanfilippo, M.R., Pellicone J.T., Damon, W.J., and King M.L. (1995). Effect of tracheostomy speaking valve on secretions, arterial oxygenation, and olfaction: A qualitative evaluation. Journal of Speech and Hearing Research, 38: 549-555.
Manzano, J.L., Lubillo, S., Henríquez, D., Martín, J.C., Pérez, M.C., and Wilson, D.J. (1993). Verbal communication of ventilator-dependent patients. Critical Care Medicine, 21(4), 512-517. doi:10.1097/00003246-199304000-00009
Mirzakhani, H., Williams, J.N., Mello, J., Joseph, S., Meyer, M., Waak, K., Schmidt, U., Kelly, E., and Eikermann, M. (2012). Muscle Weakness Predicts Pharyngeal Dysfunction in Symptomatic Aspiration in Long-term Ventilated Patients. Anesthesiology, 119(2): 389-397.
Passy V., Baydur A., Prentice, W., and Darnell-Neal, R. (1993). Passy-Muir tracheostomy speaking valve on ventilator-dependent patients. Laryngoscope, June; 103(6): 653-8.
Perme C., and Chandrashekar R.K. (2008). Managing the patient on mechanical ventilation in ICU: early mobility and walking program. Acute Care Perspectives, 17(1): 10-15.
Prigent, H., Lejaille, M., Terzi, N., Annane, D., Figere, M., Orlikowski, D., and Lofaso, F. (2011). Effect of a tracheostomy speaking valve on breathing–swallowing interaction. Intensive Care Medicine, 38(1), 85-90. doi:10.1007/s00134-011-2417-8
Rodrigues K.A., Machado, F.R., Chiari B.M., Rosseti H.B., Lorenzon P., and Goncalves MIR (2015). Swallowing rehabilitation of dysphagia tracheostomized patients under mechanical ventilation in intensive care units: a feasibility study. Revista Brasileira de Terapia Intensiva, 27(1): 64-71.
Sapienza, C.M., and Troche, M.S. (2012). Respiratory muscle strength training: Theory and practice. San Diego: Plural Pub.
Sasaki, C., Suzuki, M., Horiuchi, Masatoshi, H., and Kirchner, J.A. (1997). The effect of tracheostomy on the laryngeal closure reflex. The Laryngoscope, 87: 1428-1433.
Sciaky A.J. (1994). Mobilizing the intensive care unit patient: pathophysiology and treatment. Physical Therapy Practice, 3(2): 69-80
Siebens, A.A., Tippett, D.C., Kirby, N., and French, J. (1993). Dysphagia and expiratory air flow. Dysphagia, 8(3), 266-269. doi:10.1007/bf01354549
Speed, L. and Harding, K.E. (2013). Tracheostomy teams reduce total tracheostomy time and increase speaking valve use: a systemtic review and meta-analysis. Journal of Critical Care, 28(2):216.
Spicher, J.E. and White D.P. (1987). Outcome and function following prolonged mechanical ventilation. Archives of Internal Medicine, 147(3): 421-425.
Sutt, A.L., Cornwel, P., Mullany, D., Kinneally, T., and Fraser, J. (2015). The use of tracheostomy speaking valves in mechanically ventilated patients in improved communication and does not prolong ventilation time in cardiothoracic intensive care unit patients. Journal of Critical Care, 30:491-494.
Yu, M. (2010). Tracheostomy patients on the ward: multiple benefits from a multidisciplinary team? Critical Care, 14:109T.
Zilberberg, M. and Shorr, A. (2008). Prolonged acute mechanical ventilation and hospital bed utilization in 2020 in the United States: implications for budgets, plant and personnel planning. BMC Health Services Research, 8:242.